Home » Posts tagged 'wellness' (Page 2)
Tag Archives: wellness
Viverae wellness primes its own pump for an EEOC wellness lawsuit
Viverae
Category: Wellness
Short Summary of Company:
“Viverae gives our clients a platform for managing healthcare costs by motivating their employees to make healthy choices. Our comprehensive wellness programs address your organization’s goals to meet your employees where they are.”
Materials Being Reviewed:
Questions for Viverae:
General: What customers have actually signed up for this and are willing to admit it?
ANS: Refused to answer
Provision #2: Since your biometrics are out of compliance with USPSTF guidelines, wouldn’t a customer be risking an EEOC lawsuit by “requiring” every employee to do this against their will, subject to a large fine?
ANS: Refused to answer
Provision #4: Isn’t this the same as saying “If you sign up for two years, we’ll give you a third year maybe at a 20% discount if you do everything perfectly, but by doing so you waive your right to cancel after one or two years” ?
ANS: Refused to answer
Provision #5: Has any customer of Viverae or any other wellness vendor with 1000 or more employees completed HRAs and submitted to biometric screens at a 100% rate, as you require in Provision #2?
ANS: Refused to answer
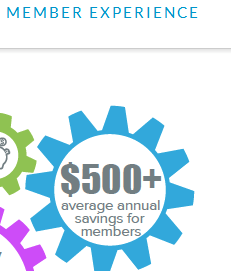
Provision #6: How could a health plan get a positive return on this program by offering people $720 apiece, when wellness-sensitive medical events account for less than $200/person in claims spend?
ANS: Refused to answer
Speaking of Provision #6, if your very own website says savings are $500/person (I’d be curious what legitimate academic research supports that), how can you guarantee savings when the cost of the incentive alone is $720?
ANS: Refused to answer
Dr. Aetna Will See You Now
Aetna
Short Summary of Intervention:
“Aetna is launching a pilot program to test the benefits of new FDA-approved, prescription weight-loss drugs combined with lifestyle support. – See more in this news release.
- Aetna Press Release
- ”Special Communication” in JAMA Internal Medicine, published by the American Medical Association
- Aetna Presentation April 17
Summary of Aetna’s key points:
Self-insured employers can sign up for this program in which Aetna will outreach to obese employees and recommend use of the drugs Belviq and Qsymia
Questions for Aetna:
You are only offering this program to self-insured employers. If this is, as your title says: a “strategy to improve health status and reduce costs,” why are you denying this program to your own fully insured members, where the cost savings would accrue directly to your own shareholders while the health status improvements would benefit your own members?
ANS: Refused to answer
Does it concern you that neither drug in your pilot is approved in Europe and that JAMA Internal Medicine says the drugs have been associated with serious harms and that these well-respected JAMA physician editorialists state that these drugs should not have been approved for use in the United States?
ANS: Refused to answer
How does your description of these drugs on your sales slide as “safe and effective” square with the question above?
ANS: Refused to answer
Why, over the course of the 70-minute webinar (for which attendees were charged $300), didn’t you mention the JAMA essay or any other safety concerns?
ANS: Refused to answer
In addition to omitting mention of the potential harms in the JAMA article, none of your materials mention that the (many) known side effects include impacts on memory, attention and language. Wouldn’t those side effects be of concern to an employer who is interested in, as your materials say, increasing the productivity of the employees taking the drugs?
ANS: Refused to answer
Does it concern you that these drugs have been by and large rejected by patients and physicians, with sales for Belviq “well below even reduced Wall Street expectations” while Qsymia has been described as ”flailing” ?
ANS: Refused to answer
Does it concern you that, of any drug on the market, Belviq has the highest ratio of payments to doctors to overall sales?
ANS: Refused to answer
Suppose an employee’s doctor won’t prescribe these drugs. Many doctors refuse to prescribe these drugs because of the side effect profiles (hence the very low sales figures). In that case, will you pressure the doctor to prescribe the drugs, get a list from the manufacturers of doctors in the area willing to prescribe the drugs and encourage the employee to switch doctors, or pressure the employer to tell the employee to drop out of this program they were just recruited into at your request? In other words, if the doctor doesn’t comply, will Aetna play doctor?
ANS: Refused to answer
Are you aware of any other health plans that will recommend name-brand drugs to members who call and say that they have obesity or any other disease?
ANS: Refused to answer
Are you aware of any other health plans that, rather than wait for members to ask for drug recommendations, outreach to members who have a disease in order to recommend proprietary name-brand drugs?
ANS: Refused to answer
Are you aware of any other health plans that outreach to members who do not have a disease, but only a high BMI, to recommend proprietary prescription drugs, especially prescription drugs that “have been associated with serious harms”?
ANS: Refused to answer
You said on your webinar that people who go off these drugs will “gradually regain weight.” In that sense, other than the $2400/year cost and “potential for serious harm,” how would this result different from any other diet, in that people who stop adhering carefully will gradually regain their weight?
ANS: Refused to answer
Any responses, apologies, retractions, changes etc. by the vendor are listed here:
June 23, 2014: Note from Ed Pezalla at Aetna, Vice President for Pharmacy Policy and Strategy: “Thank you for reaching out and inviting additional dialogue. We have a new article about the program that addresses many of your questions. We expect to publish the article in the next week or so in Aetna’s Health Section. I can send you a link once it is posted.”
August 11, 2014: Ed Pezella sent an article that would “answer some of the questions“. I am having trouble locating the answers in that article but perhaps that’s because I can’t find my reading glasses.
October 2015: Qsymia sales still “flailing.” May be off the market by 2017. Belviq struggling as well. Aetna could have avoided this entire embarrassment in the first place if they had simply asked us if pitching obesity drugs to its customers was a good idea. Come to think of it, they didn’t have to ask us. They could have asked anyone with an IQ over 80.
May 2016: STATNews finds that obesity drugs — specifically these two — have been abject failures in the marketplace.